Batten Disease Gene Therapy
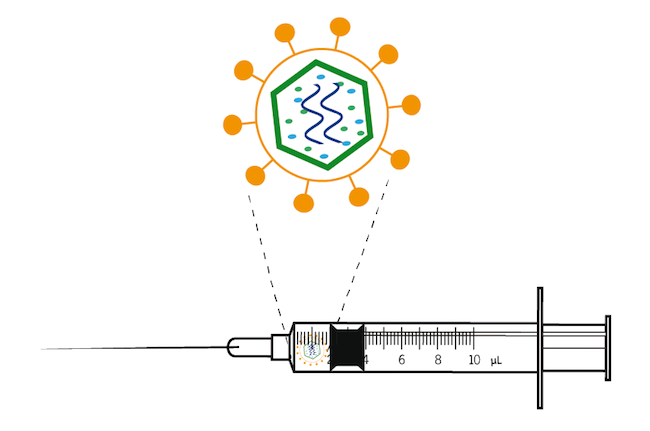
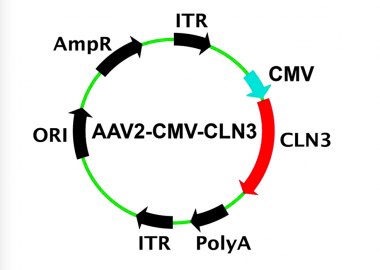
Batten disease gene therapy. Scientists are using a modified safe virus to deliver a functioning gene to the brain with hopes the replacement gene will take over or restore the mutated genes normal function. Much of the NINDS research on Batten disease focuses on developing a better understanding of the disease gene therapy and the development of drugs to treat the disorder. Amicus AAV-CLN6 gene therapy uses an inactive adeno-associated virus AVV as a vector to deliver a functional copy of the CLN6 gene into the cells of patients with CLN6 Batten disease.
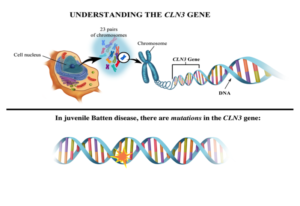
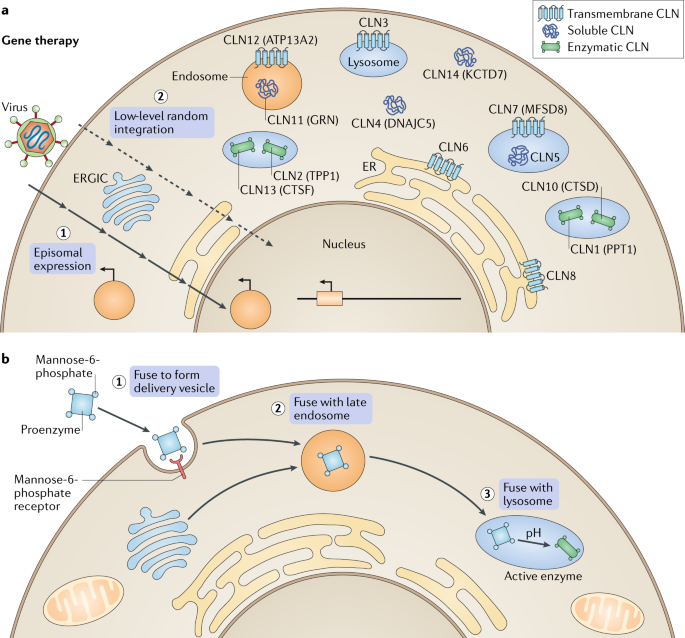
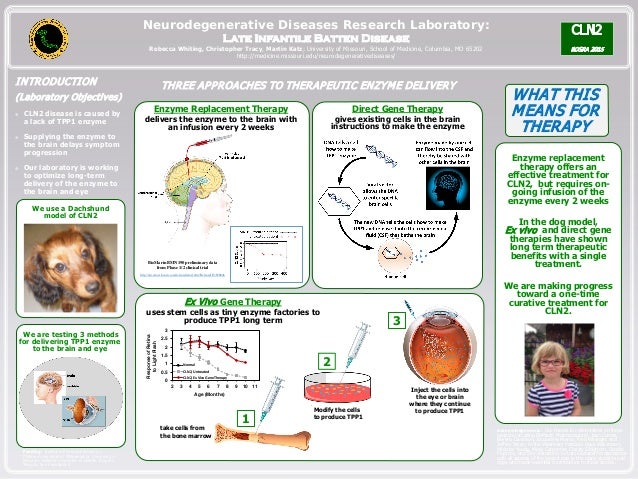
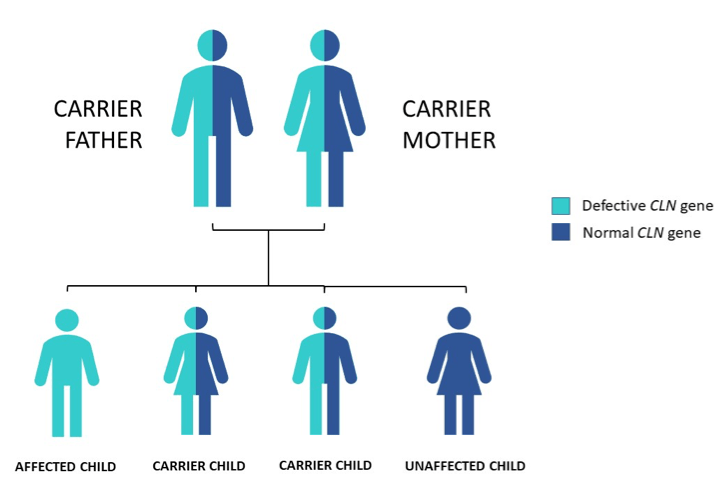
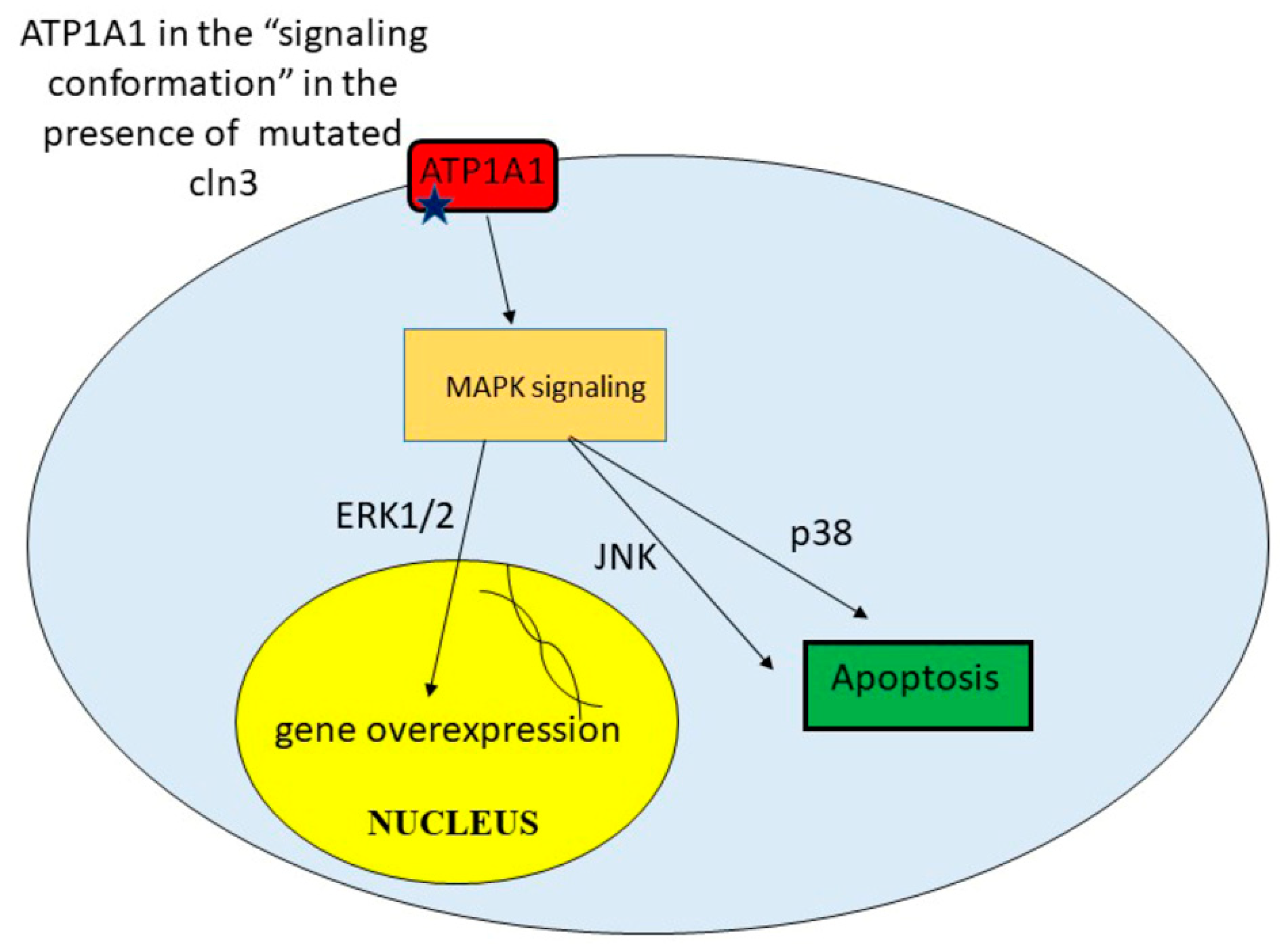
Batten disease occurs when at least one of the thirteen known ceroid lipofuscinosis CLN genes is mutated. The FDA has approved Brineura cerliponase alfa as a treatment for a specific form of Batten disease. Food and Drug Administration FDA.
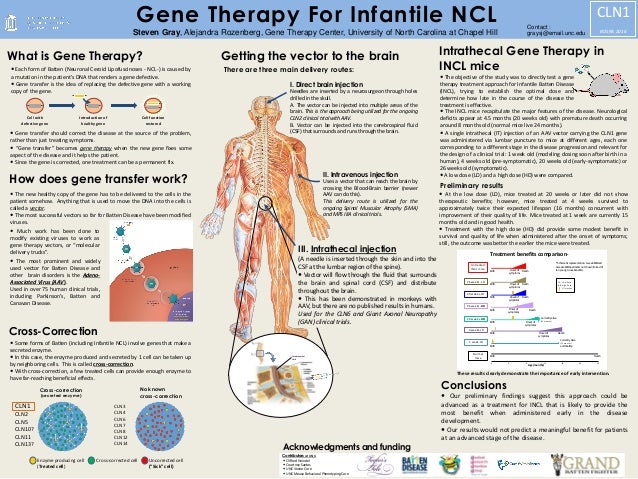
The therapy is delivered intrathecally that is injected directly into the spinal canal. Amicus Therapeutics has shared interim data on a Batten disease gene therapy it acquired last year. Using a mouse model of the disease they found some effectiveness in using stand-alone gene therapy but no detectable increase in palmitoyl-protein thioesterate-1 PPT1 activity in the brain using bone marrow transplants alone.
Intracranial delivery of AAV9 gene therapy partially prevents retinal degeneration and visual deficits in CLN6-Batten disease mice Molecular Therapy - Methods Clinical Development Vol. In the study published Dec. Brineura is the first FDA-approved treatment to slow loss of walking ability ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 CLN2 also known as tripeptidyl peptidase-1 TPP1 deficiency.
On May 21 Abeona Therapeutics announced the go-ahead from the Food and Drug Administration FDA for a clinical trial to test a gene therapy for a form of Batten disease called CLN1 disease aka infantile neuronal ceroid lipofuscinosis. Ronald Crystal chairman of the Department of. One-and-done gene therapy - Injecting a shot of genes into the brains ventricular plumbing system may be an effective long term method for treating neurological disorders.
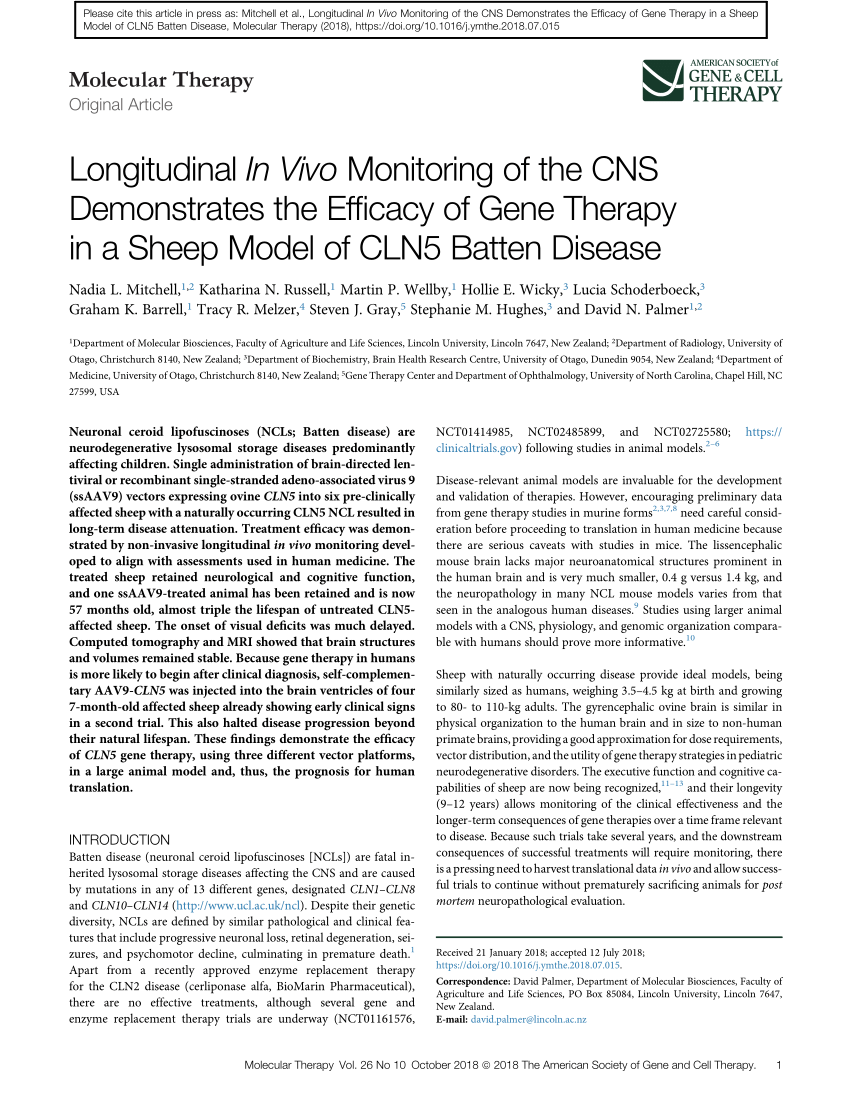
Delivery of this therapy into the brain of CLN6-Batten mice prevented most neurodegenerative symptoms with treated mice living a full lifespan. Scientists are combining gene therapy with bone marrow transplantation to treat infantile Batten disease. Batten disease may benefit from gene therapy NIH-funded animal study suggests one-shot approach to injecting genes.
A single injection into the brain of an investigational gene therapy now in clinical trials partly prevented retinal degeneration and vision loss in a mouse model of CLN6 Batten disease a study. CLN6-Batten disease is a fatal lysosomal storage disorder that primarily affects children.
Much of the NINDS research on Batten disease focuses on developing a better understanding of the disease gene therapy and the development of drugs to treat the disorder.
On May 21 Abeona Therapeutics announced the go-ahead from the Food and Drug Administration FDA for a clinical trial to test a gene therapy for a form of Batten disease called CLN1 disease aka infantile neuronal ceroid lipofuscinosis. The study has generated early evidence that. Regenxbio plans to provide updates on its RGX-181 and RGX-381 gene therapy candidates for late-infantile neuronal ceroid lipofuscinosis type 2 CLN2 disease one of the most common forms of Batten disease in the second half of this year. Batten disease a common name for a rare class of diseases called neuronal ceroid lipofuscinoses NCLs affects an estimated 2-4 out of every 100000 children in the United States. Delivery of this therapy into the brain of CLN6-Batten mice prevented most neurodegenerative symptoms with treated mice living a full lifespan. CLN6-Batten disease is a fatal lysosomal storage disorder that primarily affects children. Scientists are using a modified safe virus to deliver a functioning gene to the brain with hopes the replacement gene will take over or restore the mutated genes normal function. Brineura is the first FDA-approved treatment to slow loss of walking ability ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 CLN2 also known as tripeptidyl peptidase-1 TPP1 deficiency. Intracranial delivery of AAV9 gene therapy partially prevents retinal degeneration and visual deficits in CLN6-Batten disease mice Molecular Therapy - Methods Clinical Development Vol.
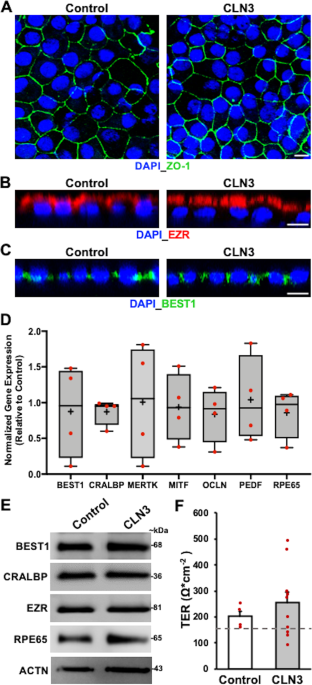
Amicus AAV-CLN6 gene therapy uses an inactive adeno-associated virus AVV as a vector to deliver a functional copy of the CLN6 gene into the cells of patients with CLN6 Batten disease. Scientists are using a modified safe virus to deliver a functioning gene to the brain with hopes the replacement gene will take over or restore the mutated genes normal function. The study has generated early evidence that. Weimer and colleagues developed an AAV9 gene therapy that was well tolerated in mice and non-human primates. 20 Imaging data on characterization of retinal autofluorescent lesions in a mouse model of juvenile neuronal ceroid lipofuscinosis CLN3 disease. Using a mouse model of the disease they found some effectiveness in using stand-alone gene therapy but no detectable increase in palmitoyl-protein thioesterate-1 PPT1 activity in the brain using bone marrow transplants alone. Batten disease occurs when at least one of the thirteen known ceroid lipofuscinosis CLN genes is mutated.




























Post a Comment for "Batten Disease Gene Therapy"